The Essential Quantum Principle That Enables Our Existence
Written on
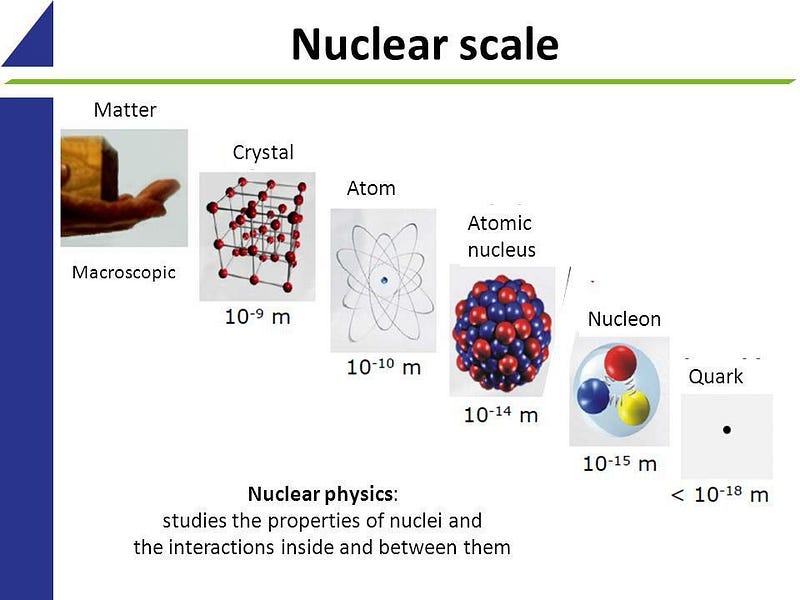
Everything on our planet consists of atoms and their fundamental components. The fascinating variety of materials we encounter would not exist without a crucial rule.
When you observe your surroundings, you can break down any object into smaller and smaller parts. Living organisms are composed of cells, which are made up of intricate molecules, and these molecules are formed from atoms. Atoms can be dissected further into their nuclei and electrons, which are the basic elements of all matter we know.
This raises an intriguing question: how do these atomic building blocks, which include fewer than 100 different types, create the vast array of molecules and objects we see? The answer lies in a largely overlooked principle of quantum mechanics: the Pauli Exclusion Principle.
When discussing quantum mechanics, most people think about its strange and counterintuitive aspects. Concepts like Heisenberg's uncertainty principle, which asserts that certain pairs of physical properties cannot be known simultaneously with absolute precision, come to mind.
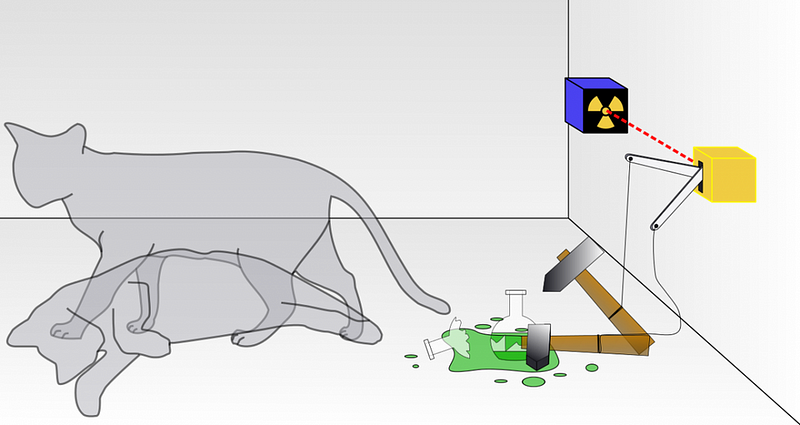
We also consider the dual nature of matter, where even individual particles like electrons or photons can exhibit interference patterns. Additionally, Schrödinger's cat illustrates how quantum systems can exist in multiple states until a definitive measurement collapses them into one outcome.
However, the Pauli Exclusion Principle, which states that no two identical fermions can occupy the same quantum state within the same system, often goes unnoticed.
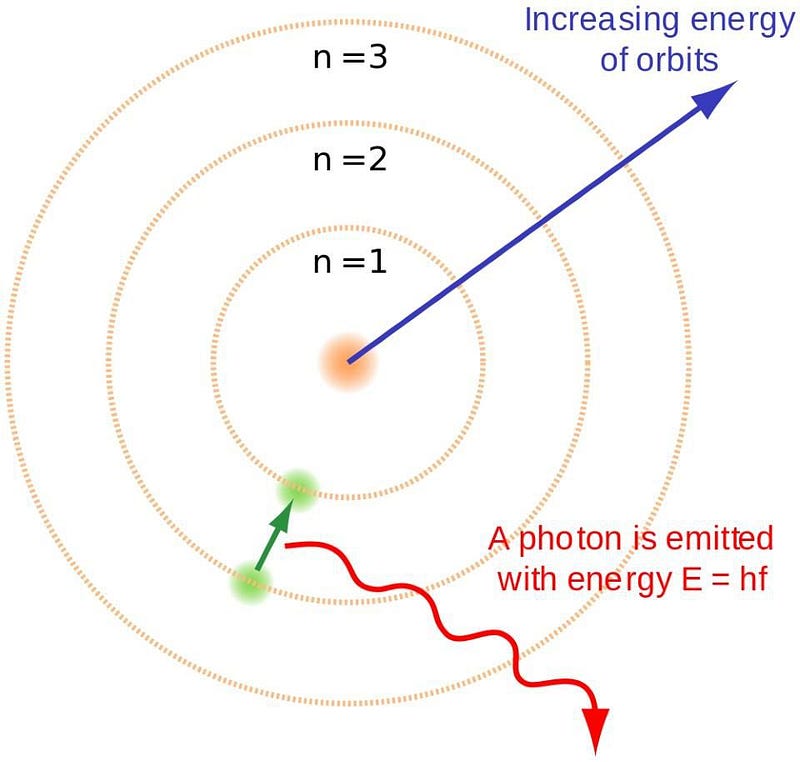
This principle is monumental. Niels Bohr's atomic model, while simplistic, effectively illustrated how electrons orbit the nucleus at defined energy levels. This model accounted for the basic structure of matter and described how electrons transition between energy levels, emitting or absorbing photons that characterize each element's spectrum.
Without the Pauli Exclusion Principle, the behavior of matter in our Universe would be entirely different. Electrons, which are fermions, are indistinguishable from one another, sharing identical mass, charge, and spin.
If this principle did not exist, there would be no limit to how many electrons could occupy the lowest energy state of an atom. Over time, all electrons would settle into this state, leading to the lowest energy orbital—the 1s orbital—being filled entirely.
Fortunately, this is not how our Universe operates. The Pauli Exclusion Principle prevents this scenario by dictating that multiple identical fermions cannot occupy the same quantum state.
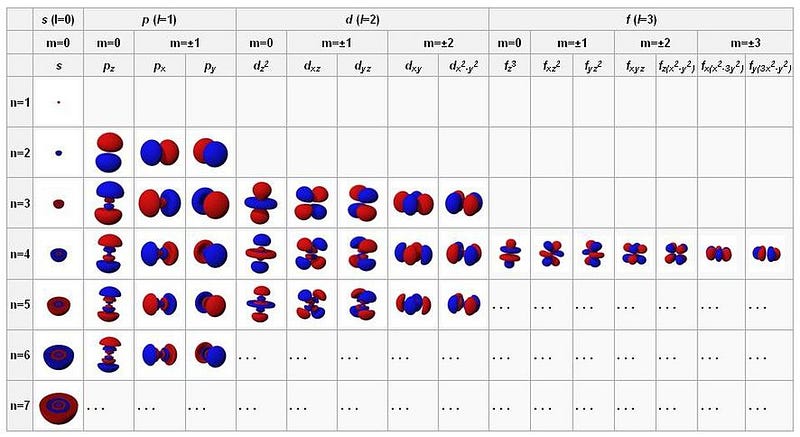
The first electron can occupy the 1s orbital, but a second electron cannot share the same quantum state. Each electron possesses unique quantum characteristics that relate to its bound state, including energy level and angular momentum.
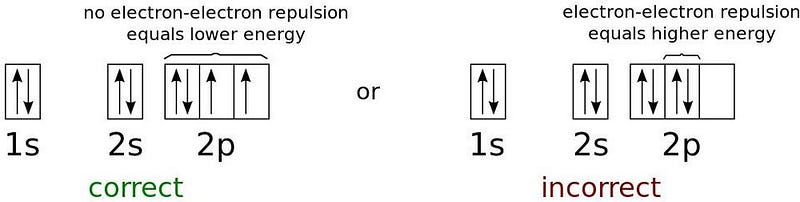
In an atom, the lowest-energy electron occupies the first energy level and can possess no angular momentum. However, the spin of the electron introduces a second possibility. Each electron has a spin of ½, meaning the electron in the 1s state can have a paired electron with an opposing spin.
This allows for two electrons to occupy the 1s orbital. Once that orbital is filled, any additional electrons must occupy higher energy levels. The second energy level, 2s, can hold two electrons, and the 2p orbital can accommodate six more.
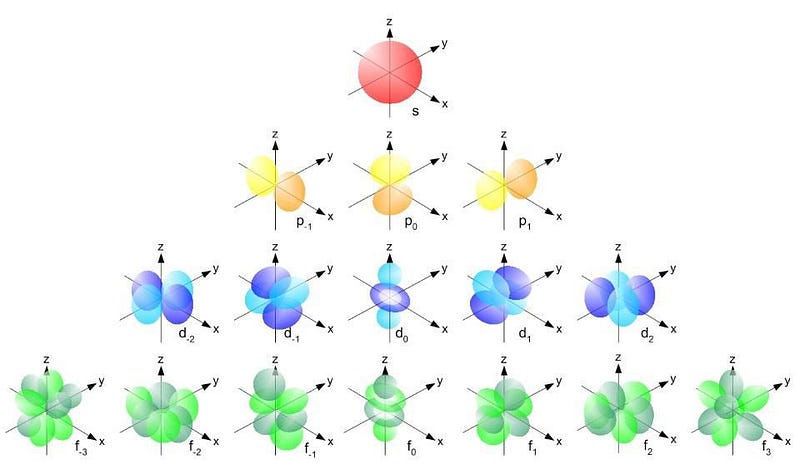
The Pauli Exclusion Principle and the specific quantum numbers create a unique structure for each atom. As we add more electrons, we must progress to higher energy levels and more complex orbitals.
The energy levels are organized as follows: - The first level (n = 1) contains only an s-orbital, accommodating just two electrons. - The second level (n = 2) consists of both s and p-orbitals, allowing for eight electrons in total. - The third level (n = 3) includes s, p, and d-orbitals, with the d-orbital permitting up to ten electrons.
Each atom on the periodic table has a distinct electron configuration due to the Pauli Exclusion Principle. The unique arrangement of outer electrons determines the physical and chemical properties of each element, resulting in a vast array of possible atomic, ionic, and molecular bonds.
No two elements can ever be identical, leading to the immense variety of molecules and complex structures that can arise from a few basic components. Each new electron requires different quantum numbers, affecting how that atom interacts with others.

Consequently, every atom presents numerous possibilities for bonding with other atoms to create chemical or biological compounds. While some configurations are energetically favorable, the variety of energy conditions in nature allows for countless combinations that challenge even the most imaginative minds.
The driving force behind this behavior is the Pauli Exclusion Principle, which restricts electrons from sharing the same quantum state. This fundamental rule prevents multiple fermions from occupying identical quantum states.
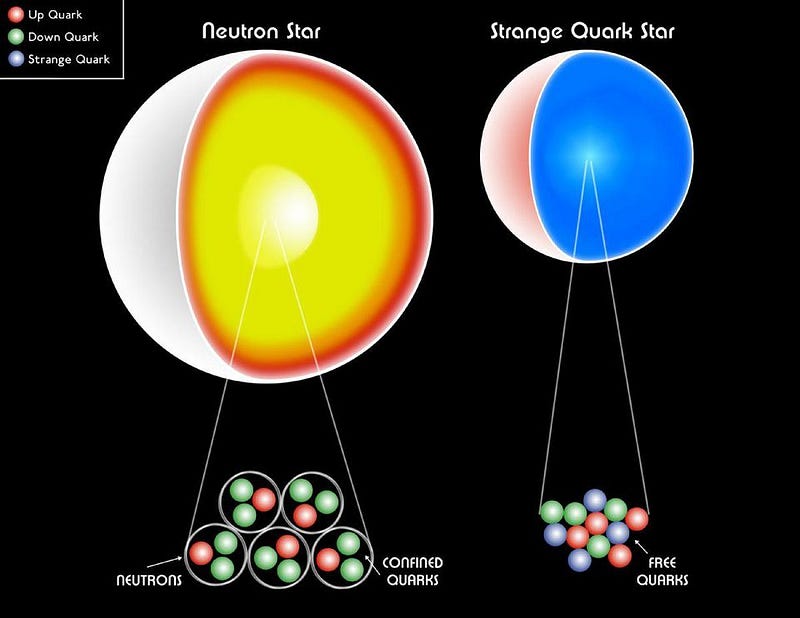
Without the Pauli Exclusion Principle, our Universe would be radically different. Atoms would possess similar properties to hydrogen, leading to simplistic structures. Stellar remnants like white dwarfs and neutron stars would collapse into black holes, and the existence of carbon-based organic compounds—the foundation of life—would be impossible.
While we may not initially consider the Pauli Exclusion Principle when contemplating the quantum rules that govern our reality, it deserves recognition. Although quantum uncertainty and wave-particle duality would still allow for some form of existence, without Pauli's rule, the complexity of molecular structures would be severely limited.
Starts With A Bang is now on Forbes and republished on Medium, thanks to our Patreon supporters. Ethan has written two books, "Beyond The Galaxy" and "Treknology: The Science of Star Trek from Tricorders to Warp Drive."